A Pennsylvania mother has sued the maker of Ozempic and Wegovy, alleging that she nearly died from taking the prescription drug and was not properly warned about the potentially distressing side effects.
Juanita Gantt said her doctor prescribed the trendy weight-loss drugs because she had a higher risk for diabetes and wanted to lose more than 20 pounds.
Her doctor initially gave her Wegovy before switching her to Ozempic. GLP-1 (or glucagon-like peptide-1) drugs are both manufactured by pharmaceutical giant Novo Nordisk.
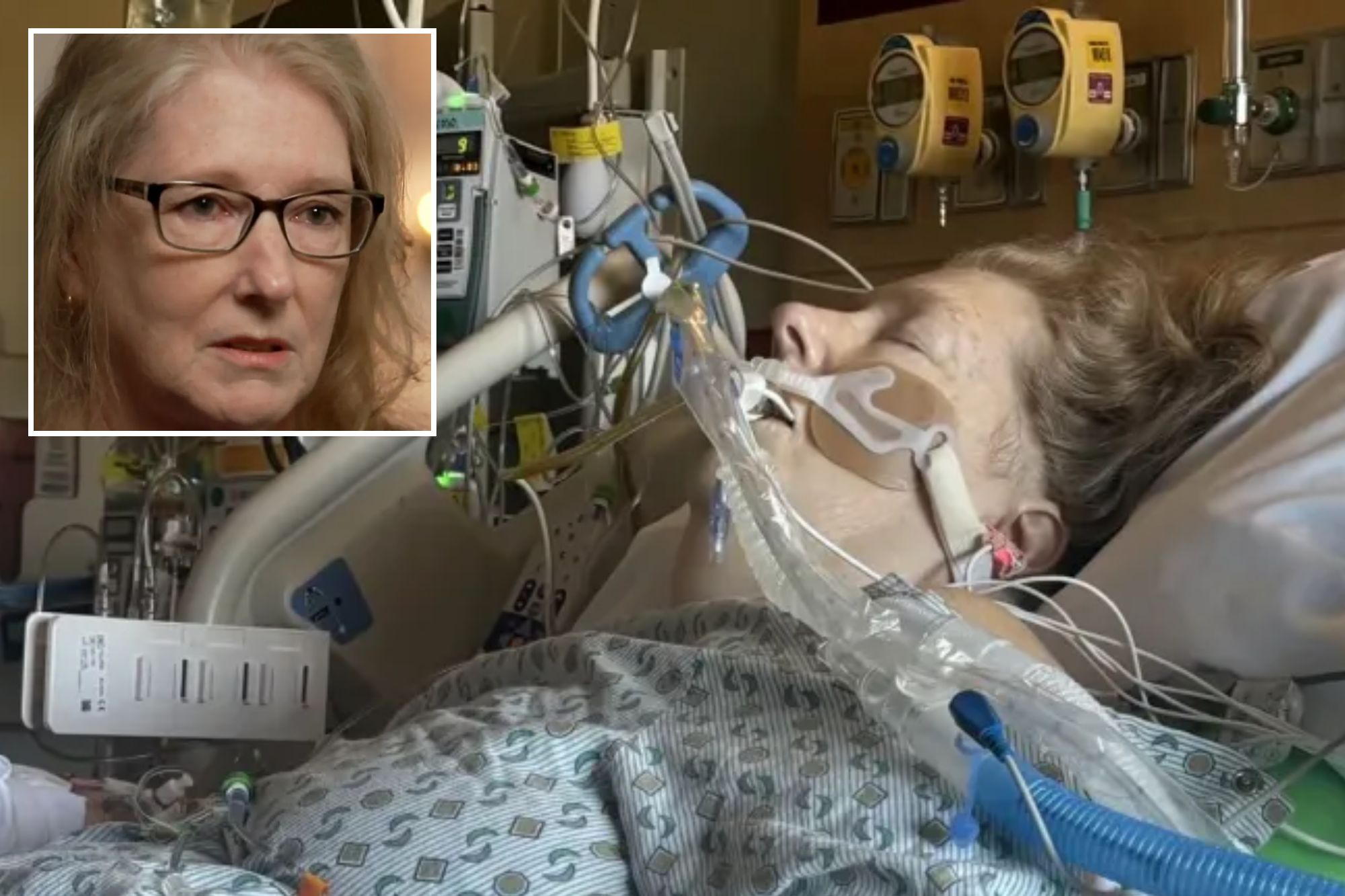
Gantt said she initially felt “fine” for the first few months of her treatment until her husband found her unconscious on the floor in October 2023.
“I had no warning that this was even a possibility,” she said in an interview with CBS News.
Doctors discovered that parts of her colon died and had to be removed, but while she was recovering from the surgery, Gantt went into cardiac arrest.
Fearing that Gantt might die, health officials went so far as to call her daughter to warn her.
“It breaks my heart that my daughter got that call,” Gantt added.
Gantt’s colon was removed as a result, and she now has to use an ileostomy bag everywhere she goes.
The horrific ordeal led Gantt to file a lawsuit against Novo Nordisk over the warning labels on their drugs. Gantt claims that the labels do not adequately warn users and doctors of serious side effects such as gastroparesis, stomach paralysis or even intestinal obstruction.
The prescription drug maker told CBS in a statement that “the allegations in the lawsuit are without merit” and that it will “vigorously defend itself against these allegations.”
The drugs were originally developed for diabetics because they stimulate the release of insulin and reduce blood sugar after meals – but in recent years, a large number of people have used the drugs to lose weight.
Ozempic warns of side effects on its website such as inflammation of the pancreas, vision changes, low blood sugar, kidney problems, serious allergic reactions, gallbladder problems and more.
In March 2024, Wegovy became “the first weight-loss drug to also be approved to help prevent life-threatening cardiovascular events in adults with cardiovascular disease and who are obese or overweight,” according to a press release from the Administration of the US Food and Drug Administration.
Ozemipc is not FDA-approved for weight management, but it is approved to help people with type 2 diabetes. However, doctors have prescribed it “off-label” in recent years due to its increased popularity for weight loss.
According to an analysis by Gallup, about 15 million Americans say they have used GLP-1 drugs to lose weight, and most people are over the age of 40.
#Pennsylvania #woman #Juanita #Gantt #claims #Ozempic #killed
Image Source : nypost.com